Figure 4 is Beers Law curve for the absorbance of an iron-salicylate complex the substance prepared in todays experiment plotted against different concentrations. The term is used in many technical areas to quantify the results of an experimental.

Q Absorbance Ratio Spectrophotometric Method For The Simultaneous Estimation Of Paracetamol And Propyphenazone In Their Combined Pharmaceutical Dosage Form Pharmatutor

Circular Dichroism Spectroscopy Units

Uv Vis Spectroscopy Purity Ratios The Bumbling Biochemist
Avobenzone is highly degraded by UV light and normal day light and it will be drastically reduced over time during the course of normal use4.

Unit for ratio of absorbance. Absorbance of Visible Light by a Food Colour Dye OBJECTIVE. Higher absorbance coefficients indicate that lower amounts of incident light will pass through the material. 2 Polynomial Functions of Higher Degree Modeling with Functions - Lesson 3.
Molar concentration also called molarity amount concentration or substance concentration is a measure of the concentration of a chemical species in particular of a solute in a solution in terms of amount of substance per unit volume of solution. But after the use of microprocessors it automatically converts transmittance into absorbance. It is given by the equation A log 10 I o I.
500 550 600 CYANMETHEMOGLOBIN 540 nm. However if you wanted to show us that sex ratio was related to population size you would use a Figure. Ideally the signal-to-background ratio should be at least 5 and preferably 10 or more for accurate quantitative work.
Similar to a monochromator but much faster the spectrometer will capture full-absorbance spectra from 220 to 1000 nm at a resolution of 1 nm in less than one secondwell. 3 torque test 8. 3 LIGHT SCATTER Flow Cytometry LASER.
How to mix 25 to 1 ratio in ml. Alternatively for samples which scatter light absorbance may be defined as the negative logarithm of one minus absorptance as measured on a uniform sample. Which of the following is the most likely explanation for the variance of the data point for the 0600 M CuSO4 solution.
Intensity is obtained using a spectrophotometer. Image will be uploaded soon The OD value for absorbance can be computed by the formula given by OD. Cells per microliter.
Complement activity enzyme units. Control degradation studies have shown that 1 hour of sunlight exposure reduces avobenzone absorbance by 364. Hence the ratio is 10010 and log 10 is one.
It is defined and expressed as the common logarithm of the ratio of incident to transmitted radiant power into a material. Thus all the units shown above are coherent SI units. 2 1 9 7 4 f x x 4.
Unlike optical absorbance the absorbance coefficient represents the optical attenuation per unit length of material typical units are 1cm. Absorbance dimensionless therefore no units. Still other sources provide protein absorbance values for 01 mgmL solutions as this unit of measure is more convenient and common for protein work.
A graph of absorbance vs concentration is called a Beers Law curve in honor of the chemist who first discovered the relationship between absorbance and concentration. The absorbance of each solution was measured and recorded. Graphs are the most common type of figure and will be discussed in detail.
Why is Absorbance the Preferred Unit Over Transmittance. The base units and those derived from them. Thouq - Instant Arabic coffee.
Absorbance A then is defined as the logarithm base 10 of the reciprocal of the transmittance. Absorbance Na Lauryl Sulfate SLS -Hemoglobin. In chemistry the most commonly used unit for molarity is the number of moles per liter having the unit symbol molL or moldm 3 in SI unit.
Pdf from ELA 9 at K12. How to mix 25 to 1 ratio in ml. Absorbance one means the 90 light has been absorbed.
Per unit volume of whole blood. This is the only base unit which has a. T PP 0.
Note that the base unit of mass is the kilogram. Is a global leader in the development manufacture and sale of life science instrumentation including imaging microscopy multi-mode detection liquid. The absorbance of a solution will change based on the wavelength that is passed through the solution.
This is a measure of how easily light passes through a material. This is because at the peak absorbance the absobance strength of light will be. Absorbance and Extinction Coefficients The ratio of radiant power transmitted P by a sample to the radiant power incident P 0 on the sample is called the transmittance T.
Absorbance units per milliliter. BMG LABTECH was the first and is the only microplate reader manufacturer to equip its instruments with a UVvis spectrometer for absorbance measurements. The unit parts per million ppm as an expression of concentration is an ambiguous term.
At times absorbance could be more than 90 in. 5 Performing Function Operations. Another name people tend to use for a logarithmic relationship factor of 10 change is order of magnitude.
The student plotted absorbance versus concentration as shown in the figure above. Bethesda unit per milliliter. Beads per 10 microliters.
Thus an instrument that can measure to 3 absorbance units has a. How to mix 25 to 1 ratio in ml. The old IR-spectra can only be found in Transmittance mode.
Any mixture in which the amount of one component is conveniently expressed s a ratio of x a. 1 unit U is the amount of. The optical density of any medium is defined as the logarithmic ratio of the intensity of incident light Io to the intensity of the transmitted light It passing through that medium.
It is important for quantitative work to operate in a range in which a plot of assay signal often absorbance versus enzyme concentration is linear. It is the measure of the capacity of a substance to observe the radiation. Examples of other types of figures are included at the end of this section.
The absorbance and transmittance are related to each other. G units per. A -log T log 1T.
Absorbance is defined as the logarithm of the ratio of incident to transmitted radiant power through a sample excluding the effects on cell walls. Absorbance is a dimensionless unit which is also called as Decadic Absorbance. Short answerUsing the maximum wavelength gives us the best results.
Figures are visual presentations of results including graphs diagrams photos drawings schematics maps etc. Prepare within 4 minutes. So it only measures the Transmittance.
An increase of 1 unit on the absorbance scale translates into a factor of 10 decrease on the T scale. Presented by AACC and NACB. 414 unit test working with functions part 1.
Absorbance can also be calculated using the ratio between the intensity of a reference sample and the unknown sample.
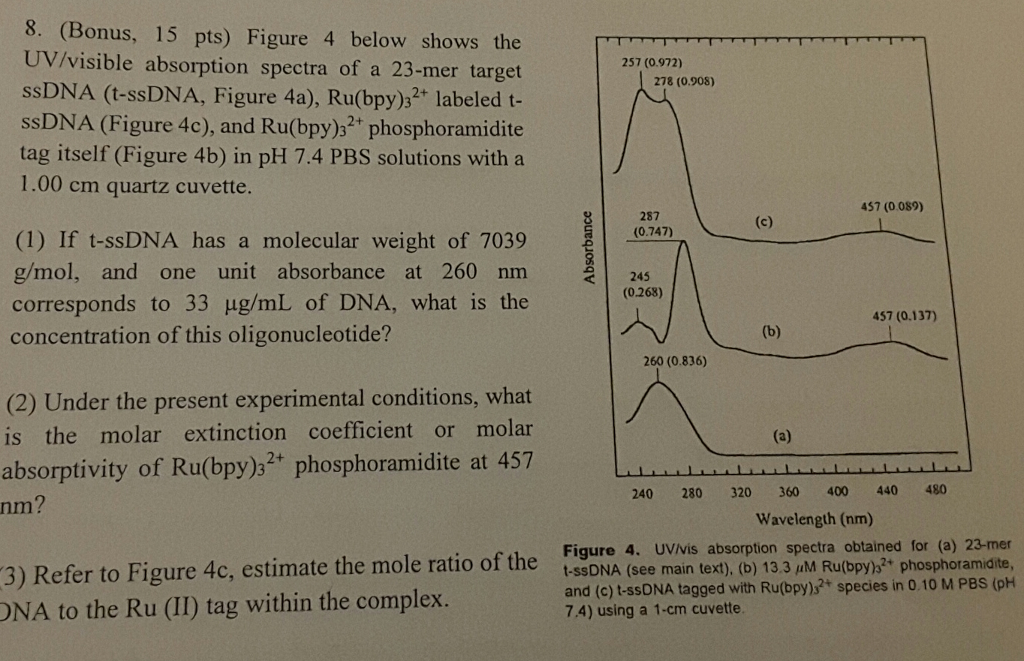
Solved Figure 4 Below Shows The Uv Visible Absorption Chegg Com

Ultraviolet Spectrophotometry An Overview Sciencedirect Topics
Tools Thermofisher Com

A Baseline Corrected Absorbance Intensity In The 990 960 Cm A1 Band Download Scientific Diagram

How To Calculate Signal To Noise Ratio Horiba

Uv Vis Spectroscopy Purity Ratios The Bumbling Biochemist

Solved I Have Been Given Absorbance Readings At 260nm And Chegg Com

Calculating Concentration Using The Beer Lambert Law Worked Example Video Khan Academy