Lacosamide is administered orally through film-coated tablets of 50 mg pink 100 mg dark yellow 150 mg salmon and 200 mg blue. Promising pharmaceutical carriers for oral delivery of poorly water-soluble drugs and peptideprotein-based therapeutics or vaccines.

Current Challenges And Future Perspectives In Oral Absorption Research An Opinion Of The Ungap Network Sciencedirect

Novel Strategies For The Formulation And Processing Of Poorly Water Soluble Drugs Sciencedirect

Lipid Based Delivery Are Lipid Based Drug Delivery Systems In Your Formulation Toolbox
It is estimated that between 40 and 70 of all new chemical entities identified in drug discovery programs are insufficiently soluble in aqueous media 1 2.

Hydrophilic drugs are often poorly absorbed when administered orally. Especially for class II low solubility and high permeability substances according to the BCS the bioavailability may be enhanced by increasing the solubility and dissolution rate of the drug in the gastro-intestinal fluids. Theophylline Caffeine 50. Marketed as Tamiflu by GileadRoche or phosphonic acid esters 4243 of poorly.
79 Volume of distribution of a drug depends on its pharmacological and physicochemical properties in particular its binding affinities. Fluoroquinolones and the oxazolidinones are well absorbed and attain high blood levels administered either orally or intravenously. The poor solubility and low dissolution rate of poorly water soluble drugs in the aqueous gastrointestinal fluids often cause insufficient bioavailability.
Diabetes2quiz How Medical Nutrition Therapy Can Improve Diabetes Management. 2 1 tsp answer 4 1 4 2 If a childs dose of a cough syrup is 34 teaspoonful and represents 14 of the adult dose calculate the corresponding adult dose. The usual maintenance dose is 200 mg daily given in divided.
Self-emulsifying drug delivery systems SEDDS can improve the oral bioavailability of poorly water-soluble drugs. Poorly absorbed from GIT Very weak bases eg. The purpose of this chapter is to provide the healthcare practitioner with an overview of the existing oral and injectable non-insulin.
Few drugs are readily absorbed in this way but for those that are such as nitroglycerin and certain steroid hormones a number of advantages may result. If the adult dose of a medication is 2 teaspoonsful tsp calculate the dose for a child if it is 14 of the adult dose. 7 Thus the excellent bioavailability after oral administration of the fluoroquinolones can obviate the need for intravenous administration in certain cases.
80 Depending on whether the drug is a lipophilic or hydrophilic molecule different models of dose adjustment are required in the obese patient. Biomedical Materials publishes original research findings and critical reviews that contribute to our knowledge about the composition properties and performance of materials for all applications relevant to human healthcare. Biopharmaceuticals and biotechnology-derived therapeutic agents face similar challenges.
3 1 3 4 3 4 12 tsp. Patients who fail to tolerate the full iv. While lifestyle changes such as dietary modification and increased physical activity can be very effective in improving glycemic control over the long-term most individuals with T2DM will require medications to achieve and maintain glycemic control.
3 tsp answer 4 4 4 1 4 1 4 NOTE. Dose should be given half the suggested oral dose. The poor solubility and low dissolution rate of poorly water soluble drugs in the aqueous gastrointestinal fluids often cause insufficient bioavailability.
Iii Tablet 1000ug - 5 - A standard tablet formulation of B 12 that is absorbed through the gastrointestinal tract. Iii Tablet 1000ug - 5 - A standard tablet formulation of B 12 that is absorbed through the gastrointestinal tract. Academiaedu is a platform for academics to share research papers.
It can also be administered by injection at a concentration of 200 mg20 mL or by oral solution at a concentration of 10 mgmL. These prodrugs are often carboxylic acid esters 34041 such as GS-4104 the ethylester of GS-4071 oseltamivir. The main disadvantages of tablets are a relatively slow onset of action because of the need to pass into the intestine and then undergo disintegration and dissolution before absorption across the gut wall the low bioavailability of poorly water-soluble drugs or poorly absorbed drugs and the local irritation of the GI mucosa that some drugs may cause.
Solid self-emulsifying drug delivery systems s-SEDDS offer several advantages including improved drug stability ease of administration and production. Oral dosage forms comprise pharmaceutical formulations taken orally for systemic effects. While this route is an easy to master quick suitable for chronic treatments and with low impact of stress on laboratory rodents there is a common concern that it may not be an acceptable route for drug administration in experimental studies.
An Introduction to Medicinal Chemistry Fifth Edition- Graham L. Availability of food the individuals lifestyle their living conditions prescribed medicationsSimilarly early results from the. Dissolution is rate limiting Permeation is rate limiting step for lipophillic drugs.
However a general statement about how distribution of drugs is changed in obesity is not possible. Iv Chewable 5000ug - 27 - A dissolvabletablet of B 12 that is absorbed through the sublingual mucosa v Liposome 1000ug - 14 - A liposome oral spray of B 12 with particle sizes of approximately 100 nm. Diabetes Prevention Program Knowler et al 2002 which was developed to compare several lifestyle self-management strategies.
Especially for class II low solubility and high permeability substances according to the BCS the bioavailability may be enhanced by increasing the solubility and dissolution rate of the drug in the gastro-intestinal fluids. Iv Chewable 5000ug - 27 - A dissolvabletablet of B 12 that is absorbed through the sublingual mucosa v Liposome 1000ug - 14 - A liposome oral spray of B 12 with particle sizes of approximately 100 nm. The latter is.
Step for hydrophilic drugs. For BCS class II drugs the dissolution step is the rate-determining factor in drug absorption. Certain agents are poorly if at all absorbed when orally administered eg penicillin G ampicillin.
Orally therapy should commence 15 minutes after the last intravenous injection with 50mg every 6 hours for 48 hours and preferably within 12 hours of the onset of chest pain. Most compounds employed in developing s-SEDDS are solid in nature with a high amount of surfactants added. Various strategies have been widely investigated to enhance the bioavailability of poorly absorbed drugs in order to increase their clinical efficacy when administered orally.
They are absorbed through the various epithelia and mucosa of the gastrointestinal tract at varying rates with the exception of drugs that are absorbed in the buccal cavity. Soluble effervescent tablets are prepared by compression and contain in addition to active ingredients mixtures of acids citric acid tartaric acid and sodium bicarbonate which release carbon dioxide when dissolved in water. The rate determining steps in absorption of orally administered drugs are.
These drugs exhibit erratic or incomplete absorption often leading to unsatisfactory drug exposure in vivo and poor bioavailability. Intraperitoneal IP route of drug administration in laboratory animals is a common practice in many in vivo studies of disease models.

What Is Drug Absorption
Drug Absorption In The Small Intestine Deranged Physiology

Poor Solubility Where Do We Stand 25 Years After The Rule Of Five American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
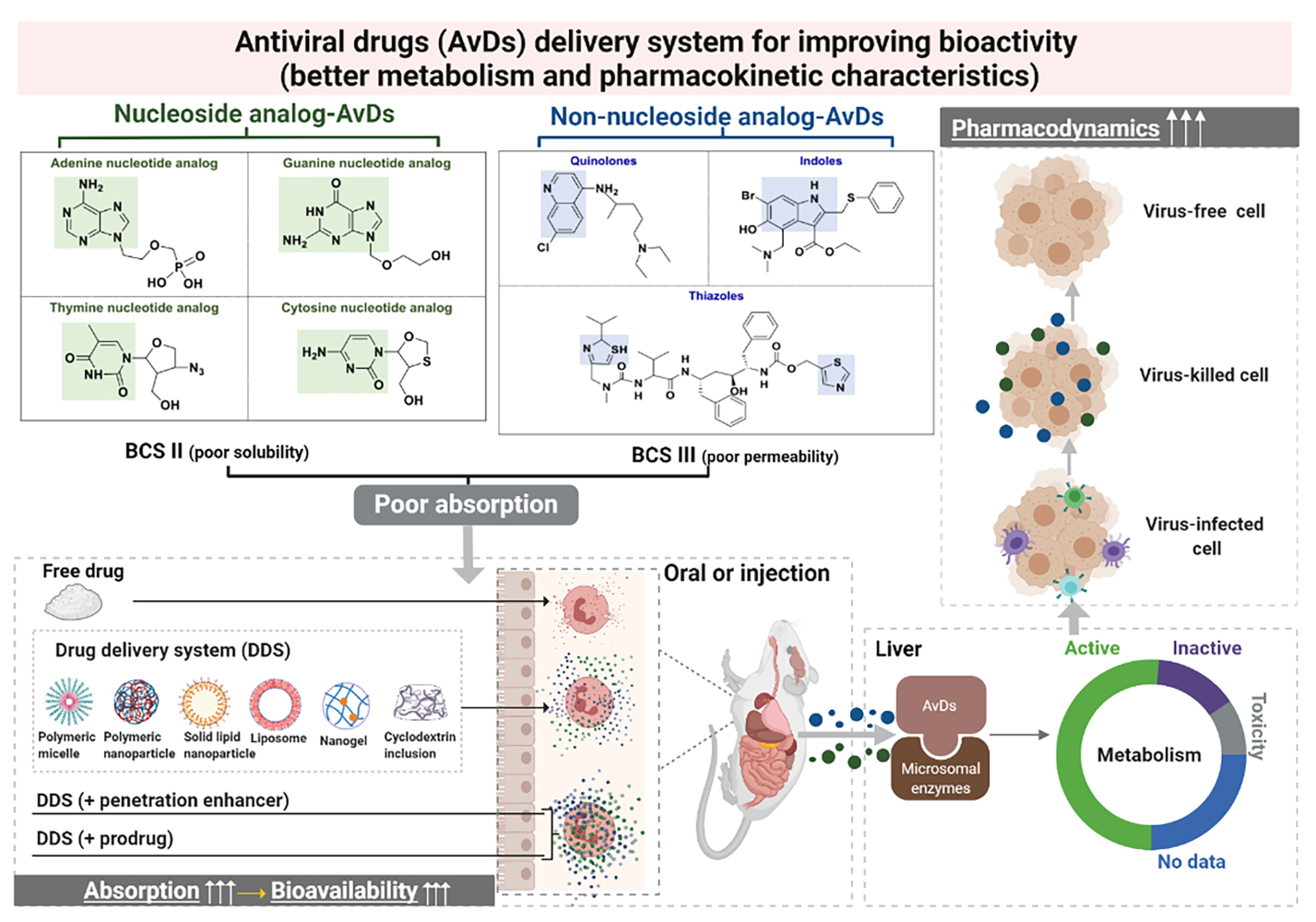
Avd Delivery System For Enhanced Bioactivity Pk Properties Ijn

Reasons For Poor Oral Bioavailability Of Poorly Water Soluble Drugs Download Scientific Diagram

Scielo Brasil Challenges To Improve The Biopharmaceutical Properties Of Poorly Water Soluble Drugs And The Application Of The Solid Dispersion Technology Challenges To Improve The Biopharmaceutical Properties Of Poorly Water Soluble Drugs

Successful Oral Delivery Of Poorly Water Soluble Drugs Both Depends On The Intraluminal Behavior Of Drugs And Of Appropriate Advanced Drug Delivery Systems Sciencedirect

Unlocking The Use Of Lipid Based Formulations With Lipophilic Salts To Address Bioavailability Challenges In Small Molecule Drug Development American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
