Absorbance is plotted on the y-axisThe command for getting an equation is Add Trendline in Microsoft Excel. We can use this plot to find the concentration of an unknown solution of Bromophenol Blue.
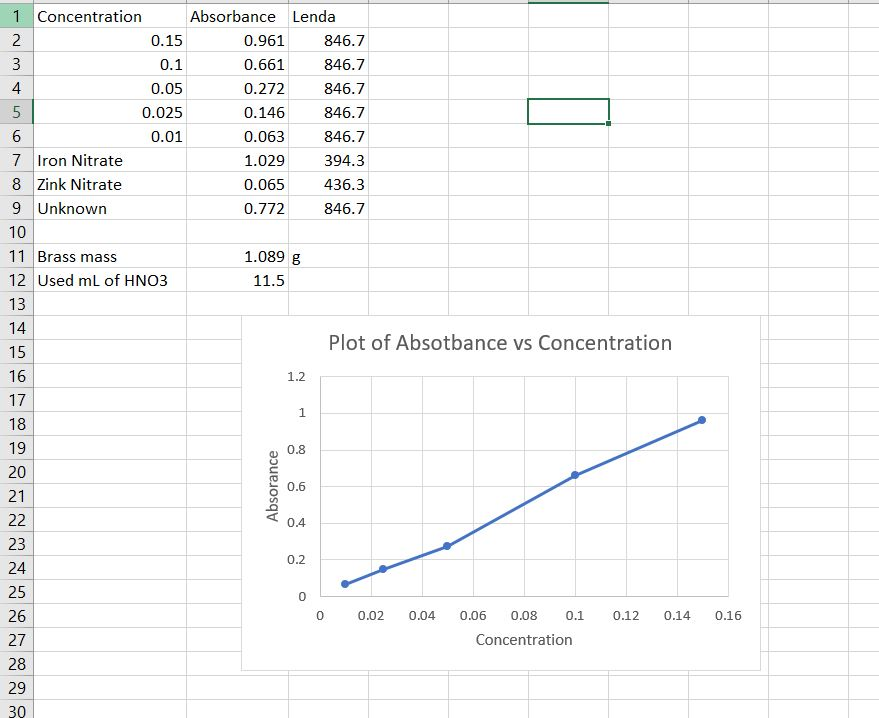
Solved Plot Absorbance Vs Concentration In Excel Note That Chegg Com

How To Create A Linear Standard Curve In Excel

How To Create A Linear Standard Curve In Excel
This is an application of regression that most people will be familiar with.
How to find unknown concentration from absorbance in excel. You can use the concentration from a titration to find pH by using pH -logH If you find an unknown concentration of HCl is 0. 5 mL of HCl has been added. From the calibration curve using graphical interpolation from the graph or mathematical methods using Beer-Lamberts Equation the corresponding value of solution concentration for the observed value of the unknown solutions absorbance can be obtained.
It is likely that the unknown will have absorbance numbers outside the range of the standard. Since concentration and absorbance are proportional Beers Law makes it possible to determine an unknown concentration of phosphate after determining the absorbance. In this instance known concentrations of a protein were measured for their absorbance at 450 nanometers in a spectrophotometer.
Z 1 2 0. Concentration is the molar absorption coefficient. You have an absorbance and you have a straight line equation that relates absorbance to concentration.
James Holler Stanley R. Well you have your calibration graph and it SHOULD look something like this all properly labeled. Now if we measure the absorbance of a sample containing an unknown amount of analyte it becomes a simple matter of substituting this value into the equation for our calibration curve and solving for the concentration.
Solving this equation for Concentration yields Concentration Signal - intercept slope where Signal is the signal reading eg. The overall goal of this lab was to make a calibration curve with a plot of absorbance vs. In this experiment a calibration curve often called a Beers Law plot.
I believe that the equation should give me direct calculation of unknown concentration by putting absorbance y in the equation ie. 00 ml of water were added to the 10. The absorbance measurements of the standard solutions are used to determine the concentration of iron in the pill solution which is used to determine the amount of iron present in the original pill.
The standard deviation in the data set. Solving for x the protein concentration for an absorbance of 06 gives. Absorbance of the unknown solution.
The relationship between concentration and absorbance is not linear. 36 and Excel pp 39-43 especially the. Concentration and be able to determine the phosphate concentrations in samples of cola surface water and other aqueous solutions of.
Where do you begin. Samples may also need to be diluted. A typical standard curve based on Beers Law.
The first worksheet abs contains data generated for a standard curve. If youre taking a science or chemistry class you may need to know how to calculate pH based off concentration instead. The equation displayed on the chart gives a means for calculating the absorbance and therefore concentration of the unknown samples.
The absorbance of the unknown solution is measured and the equation is used to find its concentration. The mean ceSO4-2 concentration. Using spectrophotometry the absorbance of the unknown solution can be determined.
In order to. Absorbance of a solution of unknown concentration can then be measured with the spectrophotometer and this value can be used in conjunction with the calibration curve to determine the concentration of this solution. Academiaedu is a platform for academics to share research papers.
Ppm calculator excel email protected. However it is a convenient method providing a rough estimation. Beers Law is considered to be most reliable for absorbance values between 01 and 1.
Unknown concentrations of a given molecule can be determined using the equation of. Cultures were diluted in fresh LB until achieve 01 OD with the corresponding antibiotic and transferred to a 96-well plate 50 µLwell. Analytical Chemistry Douglas A.
If Beers Law holds a calibration curve is a straight line for which we can obtain an equation from a regression analysis. As you can see by the Linear plot number above a linear regression does not provide a good method for comparing the protein standards to the unknown samples. The dilluted sample gives an absorbance of 0432.
1 IFTHENELSE 2 WHILEDO called by some DOWHILE 3 DOUNTIL 4 CASE Because of the lack of block structure it is necessary to use statement labels in the simulation of the standard figures. Com In this section the conventions for simulating the following standard structure figures will be outlined. The median ceSO4-2 concentration.
What is the concentration of the initial unknown. Absorbance of samples with unknown concentration Standards are serially diluted with Sample Diluent. 1In excel the charts you will find different type of graphs.
Create a scatter plot showing both the College 1 and College 2. You can plot the concentration versus absorbance then perform linear regression to find the formula that relates concentration and absorbance. How to calculate unknown concentration from standard curve.
Once the absorbance of each of the four solutions is known use Microsoft Excel to plot absorbance on the y-axis and concentration on the x-axis. For example if the absorbance of an unknown solution was found to. Here X is the unknown concentration which you need to find.
During 8h the absorbance at OD600 and fluorescence excitation 584 nm and emission 607 nm were measured with intervals of 1 hour. Examining the mathematical form of the Beer-Lambert Law the molar extinction coefficient or molar absorptivity is a constant for. Since sample diluent has absorbance even when there is no protein present at the detection wavelength we run blank well with sample diluent in the assay to get the background OD.
In Graph 1 x is concentration and y is absorbance so one must rearrange the equation to solve for x and enter the absorbance of the measured unknown. X unknown Concentration mM Y Absorbance at 420 nm 0. The Wireless Spectrometer from PASCO is specifically designed for modern chemistry biology and physics labs.
Re-calculate the following values using Excel excluding the outliers. Click here for a fill-in-the-blank OpenOffice spreadsheet that does this for you. Samples were always made in triplicates and a blank of LB.
With Bluetooth and USB connectivity students can quickly connect from their device or computer using PASCOs free Spectrometry Software. Download this weeks Excel workbook HERE. Read Section IV in the Techniques section to find out more about straight line graphs p.
Comparison Of Calibration Curve Fitting Methods In Absorption Spectroscopy
E X C E L Maple Programming
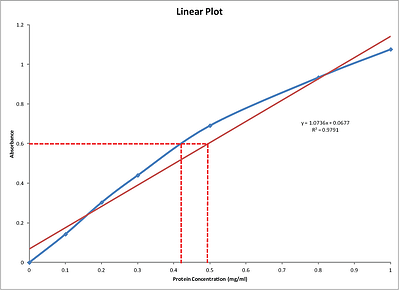
Bradford Protein Assay Calculation Of An Unknown Standard
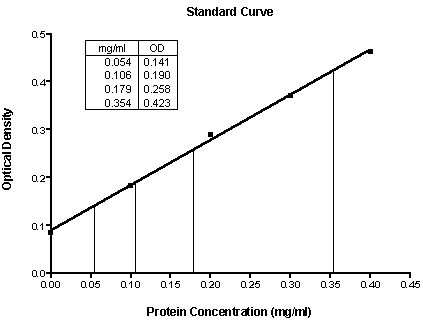
Prism 3 Calculating Unknown Concentrations Using A Standard Curve Faq 1753 Graphpad
Worksheet For Analytical Calibration Curve

Chem301 Tutorial Calculations With Excel
Ccri Edu

Chem301 Tutorial Calculations With Excel
